Cuprous Iodide
Specifications
| Item | Index | Index | Index |
| Assay(asCuI)% | ≥99.5 | ≥99.0 | ≥99.0 |
| Insol. in Hcl % | ≤0.01 | ≤0.02 | ≤0.05 |
| Sulphates(asSO4)% | ≤0.01 | ≤0.02 | ≤0.02 |
| Alkali metals and alkaline-earth metals(asSO4)% | ≤0.1 | ≤0.2 | ≤0.2 |
| Fe% | ≤0.003 | ≤0.005 | ≤0.01 |
Packing& Storage
| Packing | 25kg cardboard drum, lined with double-layer plastic bag | |||||||
| Storage | 20℃, 2 years. | |||||||
| Shipping | Room temperature in China; may vary elsewhere | |||||||
General Information
1.1 Chemical & Physical Properties
| Common Names | Cuprous iodide | ||||||
| Structure | 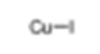 |
||||||
| CAS No. | 1335-23-5 | Boiling Point (℃) | N/A | ||||
| Molecular Weight | 190.45000 | Melting Point (℃) | N/A | ||||
| Appearance | White crystal or offwhite powder | Vapor Specific Gravity | N/A | ||||
| HS Code | 2827600000 | Flash Point (℃) | N/A | ||||
| Solubility | soluble in concentrated sulfuric acid | Autoignition Temperature (℃) | N/A | ||||
1.2 Safety Information
| Safety Phrases | 22-24/25-26-61 | |
| RIDADR | N/A | |
| WGK Germany | N/A | |
| Packaging Group | N/A | |
| Hazard Class | N/A | |
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1.3 Synthetic Route
The aqueous solution of NaHSO3 (0.25 g, 0.21 mmol) and NaI (0.6 g, 0.4 mmol) and NaI (0.6 g, 0.4 mmol) were added to the aqueous solution of CuSO4·5H2O (1 g, 0.4 mmol) under an argon atmosphere and vigorously stirred, resulting in a shallow pink solid. The precipitate is filtered under vacuum and washed with H2O, anhydrous EtOH, and anhydrous Et2O. The whole process is as fast as possible. It is then vacuum dried to obtain a light pink solid cuprous iodide
