light cuprous oxide
Specifications
| Item | Index |
| Top grade | |
| Appearance | red powder |
| Total reduction rate (calculated as Cu2O)%≥ | 98.0 |
| Metal copper (Cu) content %≤ | 1.0 |
| Cuprous oxide (Cu2O) content %≥ | 97.0 |
| Total copper (Cu) content%≥ | 87.0 |
| Chloride (Cl) content%≤ | 0.1 |
| Sulfate (calculated as SO4) %≤ | 0.1 |
| Moisture %≤ | 0.5 |
| Acetone soluble matter %≤ | 0.5 |
| Reduction of reduction rate after stability test %≤ | 2.0 |
| Residue on Sieve (45μm)%≤ | 0.3 |
| Nitric acid insoluble matter on 75μm sieve %≤ | 0.05 |
| Particle size distribution | D50, 2-4um, D90 5-7um |
Packing& Storage
| Packing | in 25kg bag | |||||||
| Storage | 20℃, 2 years. | |||||||
| Shipping | Room temperature in China; may vary elsewhere | |||||||
General Information
1.1 Chemical & Physical Properties
| Common Names | cuprous oxide | copper,hydrate | ||||||
| Structure | 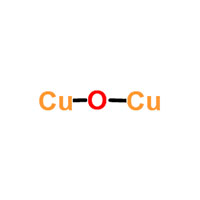 |
||||||
| CAS No. | 1317-39-1 | Boiling Point (℃) | 1800 °C | ||||
| Molecular Weight | 143.091 | Melting Point (℃) | 1232 °C | ||||
| Appearance | Red-brown powder | Vapor Specific Gravity | 4.9 | ||||
| HS Code | N/A | Flash Point (℃) | 1800°C | ||||
| Solubility | Insoluble in water and alcohol, soluble in hydrochloric acid, ammonium chloride, ammonia, slightly soluble in nitric acid. Dissolved in hydrochloric acid to generate white cuprous chloride crystalline powder. | Autoignition Temperature (℃) | N/A | ||||
1.2 Safety Information
| Safety Phrases | S22-S60-S61-S16-S7 | |
| RIDADR | UN 3077 9/PG 3 | |
| WGK Germany | 3 | |
| Packaging Group | III | |
| Hazard Class | N/A | |
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1.3 Synthetic Route
After removing impurities, dry copper powder is mixed with copper oxide, and sent to the calcined furnace to be heated to 800~900 °C to calcide into cuprous oxide. After taking out, the mechanical impurities are absorbed by magnets, and then crushed to 325 mesh to obtain a finished product of cuprous oxide. If copper sulfate is used as raw material, the copper in copper sulfate is first reduced with iron, and the subsequent reaction steps are the same as those using copper powder as raw material. 1317-39-1 preparation 2. Glucose reduction method mixes copper sulfate solution with glucose and adds sodium hydroxide solution for reaction to generate cuprous oxide, which is filtered, rinsed, dried and crushed to obtain cuprous oxide products.
