Cobaltous Nitrate
Specifications
| Item | Index | Index | Index | Index |
| Reagent grade A.R. | Reagent grade C.P. | Catalyst grade | Technical grade | |
| Assay % | ≥99 | ≥97 | ≥99 | ≥99 |
| Water insoluble% | ≤0.005 | ≤0.01 | ≤0.005 | ≤0.005 |
| Chlorides(Cl) % | ≤0.005 | ≤0.01 | ≤0.005 | ≤0.005 |
| Sulphate(SO4)% | ≤0.005 | ≤0.02 | ≤0.005 | ≤0.005 |
| Mn% | ≤0.005 | ≤0.02 | ≤0.005 | ≤0.01 |
| Fe% | ≤0.0005 | ≤0.003 | ≤0.0005 | ≤0.001 |
| Cu% | ≤0.002 | ≤0.01 | —— | —— |
| Ni% | ≤0.05 | ≤0.5 | ≤0.05 | ≤0.05 |
| Zn% | ≤0.05 | ≤0.1 | ≤0.05 | ≤0.05 |
| Alkali metals and alkaline-earth metals(As sulphates) % | ≤0.1 | ≤0.5 | ≤0.1 | ≤0.1 |
| Ammonium(NH4)% | ≤0.2 | —— | ≤0.2 | ≤0.2 |
Packing& Storage
| Packing | 25kg cardboard drum, lined with double-layer plastic bag | |||||||
| Storage | 20℃, 2 years. | |||||||
| Shipping | Room temperature in China; may vary elsewhere | |||||||
General Information
1.1 Chemical & Physical Properties
| Common Names | Cobaltous Nitrate | Cobalt nitrate hexahydrate | ||||||
| Structure | 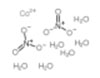 |
||||||
| CAS No. | 10026-22-9 | Boiling Point (℃) | N/A | ||||
| Molecular Weight | 291.035 | Melting Point (℃) | 55 °C(lit.) | ||||
| Appearance | Red crystals | Vapor Specific Gravity | N/A | ||||
| HS Code | 28342920 | Flash Point (℃) | N/A | ||||
| Solubility | Soluble in water | Autoignition Temperature (℃) | N/A | ||||
1.2 Safety Information
| Safety Phrases | S17-S36/37-S60-S61-S45-S36/37/39-S26-S22 | |
| RIDADR | UN 1477 5.1/PG 2 | |
| WGK Germany | 2 | |
| Packaging Group | III | |
| Hazard Class | 5.1 | |
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1.3 Synthetic Route
Nitric acid is added to cobalt hydroxide or cobalt phosphate, and recrystallized after reaction. Or use metal cobalt to react with nitric acid, add cobalt phosphate, adjust the pH value, filter to remove iron hydroxide, and then use nitric acid acidification, evaporation crystallization, can be prepared cobalt nitrate.
Synthesis method: add 30%~32% nitric acid to the acid-resistant reactor with stirring, slowly add metal cobalt particles under stirring for reaction, after the reaction is close to complete, steam is used to heat out nitrogen oxide gas, the solution is sent to the clarifier, cobalt carbonate is added under stirring to adjust the solution to pH 5 or so, kept warm at 80~90 °C for 1 day, filtered while hot to remove iron hydroxide precipitate, the filtrate is acidified to pH >1 with nitric acid, and then evaporated and concentrated to the relative density of the solution 1.6~1.7, and separated by cooling crystallization and centrifugation. Prepared cobalt nitrate finished product …
